BMRB Entry 17502
Click here to enlarge.
PDB ID: 2la4
Entry in NMR Restraints Grid
Validation report in NRG-CING
Chem Shift validation: AVS_full, LACS
BMRB Entry DOI: doi:10.13018/BMR17502
MolProbity Validation Chart
NMR-STAR file interactive viewer.
NMR-STAR v3 text file.
NMR-STAR v2.1 text file (deprecated)
XML gzip file.
RDF gzip file.
All files associated with the entry
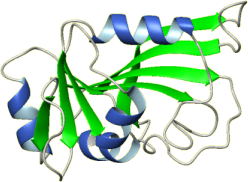
Title: NMR STRUCTURE OF THE C-TERMINAL RRM DOMAIN OF POLY(U) BINDING 1 PubMed: 21931728
Deposition date: 2011-03-01 Original release date: 2012-03-01
Authors: Santiveri, C.M.; Mirassou, Y.; Rico-Lastres, P.; Martinez-Lumbreras, S.; Perez-Canadillas, J.M.
Citation: Santiveri, Clara; Mirassou, Yasmina; Rico-Lastres, Palma; Martinez-Lumbreras, Santiago; Perez-Canadillas, Jose. "Pub1p C-terminal RRM domain interacts with Tif4631p through a conserved region neighbouring the Pab1p binding site" PLoS One 6, e24481-. (2011).
Assembly members:
NUCLEAR_AND_CYTOPLASMIC_POLYADENYLATED_RNA-BINDING_PUB1, polymer, 101 residues, 11295.992 Da.
Natural source: Common Name: baker Taxonomy ID: 4932 Superkingdom: not available Kingdom: not available Genus/species: Eukaryota Fungi
Experimental source: Production method: recombinant technology Host organism: ESCHERICHIA COLI
Entity Sequences (FASTA):
NUCLEAR_AND_CYTOPLASMIC_POLYADENYLATED_RNA-BINDING_PUB1: GSQTIGLPPQVNPQAVDHII
RSAPPRVTTAYIGNIPHFAT
EADLIPLFQNFGFILDFKHY
PEKGCCFIKYDTHEQAAVCI
VALANFPFQGRNLRTGWGKE
R
- assigned_chemical_shifts
| Data type | Count |
| 13C chemical shifts | 451 |
| 15N chemical shifts | 100 |
| 1H chemical shifts | 697 |
Additional metadata:
Related Database Links:
| PDB | 2LA4 |
| DBJ | GAA26078 |
| EMBL | CAA95877 CAY82177 |
| GB | AAA02808 AAC37348 AAC37364 AHY77075 AJP41312 |
| REF | NP_014382 |
| SP | P32588 |
| TPG | DAA10528 |
Download simulated HSQC data in one of the following formats:
CSV: Backbone
or all simulated shifts
SPARKY: Backbone
or all simulated shifts