|
Biological Magnetic Resonance Data BankA Repository for Data from NMR Spectroscopy on Proteins, Peptides, Nucleic Acids, and other Biomolecules |
Member of
|
Calmodulin Function |
|||||
| |||||
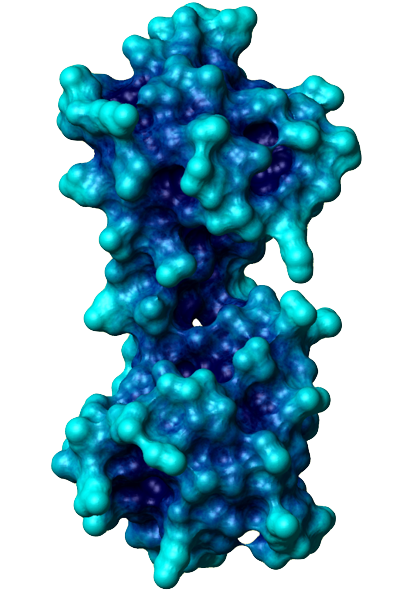
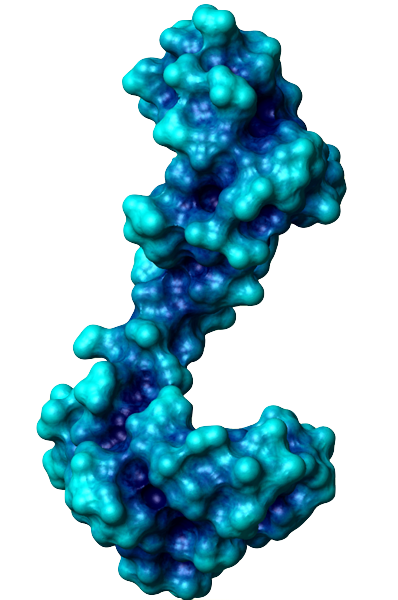
The general function of CaM is to bind calcium ions and then bind a target protein, affecting its activity. The binding of calcium is achieved by the 4 EF-hand domains. A basic EF hand consists of two perpendicular alpha helices with a 12-residue loop region between them. Residues 1, 3, 5, 7, 9 and 12 of the loop region are responsible for calcium binding. Each of these residues (with the exception of 12) contributes a sidechain or backbone oxygen atom necessary for calcium binding; residue 12, which is usually a Glu or Asp, contributes both oxygens from its side chain carboxylic acid group. In a calcium-bound EF-hand, calcium ions are consequently bound to 7 oxygen atoms in a pentagonal bipyramidal configuration (like the molecule IF7). Calcium ions usually bind a pair of EF-hands at a time, so CaM is typically bound to 0, 2, or 4 calcium ions simultaneously.
Calcium binding causes CaM to undergo a conformational change, allowing Ca2+-CaM to bind to its targets, eliciting certain responses. Specifically, the binding of calcium exposes CaM's hydrophobic regions in the globular domains so they are localized on the surface and not the protein's interior. These hydrophobic surfaces then interact with the hydrophobic regions of CaM's target proteins. When binding to targets, CaM acts as a pair of pliers, clamping down on these non-polar regions. This is made possible because the alpha helix connector is very flexible and allows CaM to wrap around targets of varying shapes and sizes. Ca2+-CaM binds many kinases, phosphatases, signaling proteins, and structural proteins affecting a wide variety of processes including neurotransmitter release, muscle contraction, metabolism, apoptosis, inflammation, membrane protein organization, and cytoskeleton movement. Apo-CaM can bind (via a different motif than Ca2+-CaM) neuroproteins, structural proteins, and signalling proteins involved in neurotransmitter production and release, nerve growth, muscle relaxation, and intracellular movement of organelles along actin filaments. | |||||
|
Previous: Introduction |